Writing about emerging trends shaping medical practice today has become as challenging as the art of practising it. It seems hard to filter noise from the value at the current dizzying pace of new healthcare technology outputs. However, looking at digital health applications through a care provider's lens, it becomes clearer that their objective needs to impact four essential indicators: access to care, relative costs, quality of care, and prevention. In a fast-expanding digital health space, one particular application domain has been gaining traction in the past few years for its capacity to mitigate all of the aforementioned indicators effectively. This new domain has been boosted by rising levels of digital literacy and maturity among healthcare professionals, in combination with higher penetration of internet and smartphone use in most countries. Welcome to the new world of Digital Therapeutics (DTx).
What are Digital Therapeutics?
DTx are evidence-based, outcome-enhancing health interventions delivered directly to the patient via software applications to prevent, manage, mitigate or treat a broad spectrum of physical diseases and mental health disorders. These interventions can be either a stand-alone app or a software adjacent to a drug prescription.
Some DTx interventions combine software with hardware such as external sensors or virtual reality (VR) goggles. DTx have become a powerful digital tool – they combine an array of mature software technologies guided by clinical evidence. DTx are not about the technology but more about how these digital interventions are being engineered and used. DTx do not undermine the efficiency of many other classic therapies that exist today but represent a solid new alternative when these interventions, for various reasons, fail to achieve the desired outcomes.
Market Size for DTx
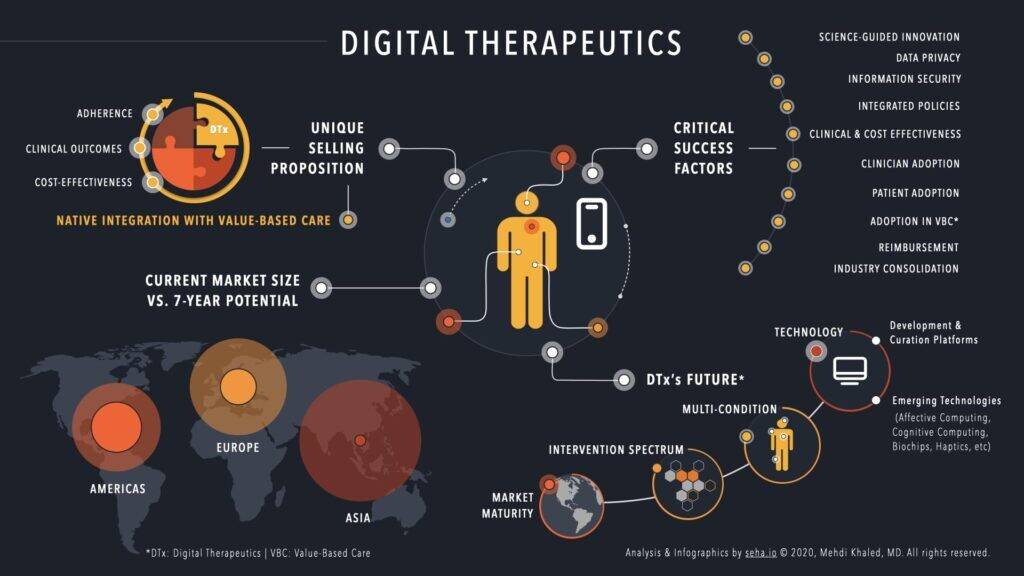
Digital Therapeutics represent today the fastest-growing segment in the digital health space. With a current market valuation of around US$2.88 billion, the estimated global market growth is between US$11,8 and US$13.8 billion by 2027, and with CAGR around 20.5%, DTx vastly outpaces any other digital health technology growth projections. Currently, the US is leading, Europe is quickly catching up, and Asia represents a great potential underdog for innovation and faster adoption.
What is DTx's Unique Selling Proposition?
Perhaps the most important but still heavily under-reported value of DTx is their native integration with value-based care (VBC) processes. Indeed, Patient-Reported Outcome Measures (PROMs) and Patient Activation Measures (PAMs) are an integral part of most, if not all, digital therapy solutions. The embedded PROMs within the digital therapy data flow are quite unique to DTx and positively feeds three critical loops in real-time:
- Adherence: DTx are patient-centric by design and therefore stage a new level of patient activation and engagement. In fact, DTx achieve this through measuring and quantifying progress by the user. This fuels emotional gains and helps the patient better understand their health condition and the immediate and long-term impact of their behaviour.
- Provide Real-World Evidence by documenting treatment efficacy and informing insurance policies.
- Personalized care.
Because of this native capability in supporting VBC frameworks and potential ease of integration in standards of care workflows, validated DTx should become the interventions of choice to drive new VBC pilots in classic healthcare settings. Having some digital therapies that are not part of a VBC bundle would almost certainly dilute the fundamental value of DTx in the long run. And although most DTx solutions have been initially designed to work in a pay-per-service setting, the shift to bundled-payment systems wouldn't require any additional engineering work by solution providers.
Kaia Health engages patients in directly managing and measuring key COPD progress indicators such as exercise capacity, health-related quality of life and level dyspnoea. This intervention alone has enhanced outcomes and reduced the cost burdens of one of the most expensive and frequent chronic disease classes. Moreover, the company's proven solution of twice reducing pain in patients with chronic musculoskeletal (MSK) conditions has won them the first spot in a newly established German government register for their DTx to be reimbursed by the social insurance scheme. This DTx-enabled MSK pain management solution represents a very welcome trend that will hopefully help rewire an often conservative medical community into rethinking first-line interventions. Today DTx are here to provide more effective, alternative and sustainable choices for drugs.
In mental health, as much as the science behind CBT is sound, most, if not all, therapeutic procedures have been designed decades ago and are based on paper and pen. DTx takes the often complex and abstract processes of CBT to a whole new palpable, visible, engaging, and most of all, measurable dimension for the patient. Moreover, the progress made by the patient is, in most cases, both self-paced and emotionally rewarding — the perfect combination for a reinforcing and positive feedback loop. Examples of such affordable, accessible and efficient DTx approaches are many. BehaVR's Addiction Recovery Augmentation or Happify's Interpersonal Psychotherapy solutions are two examples that underscore the superior and fast outcomes of DTx interventions in this field.
Regulatory Frameworks
Regulations for DTx are still in the infancy stage. However, today, not all DTx applications require regulatory clearance. The requirement for a DTx regulatory approval or clearance depends on two factors: the risk level presented by the DTx and/or the validation of claims through clinical evaluations.
The international standard IEC 62304 Medical Device software is the primary framework for requirements for developing and maintaining medical software. It covers software embedded in a medical device or an integral part of it and software that is a medical device itself (so-called stand-alone software or software as a Medical Device (SaMD)). The corresponding European version of this standard, EN 62304, includes a list of harmonized standards under the Medical Device Directive MDD (93/42 EEC). ISO 14971:2019 is now expected to complement the Medical Device Regulation MDR (EU 2017/745) to harmonize the risk management standards. By way of comparison, the FDA has already listed this ISO standard under "Recognised Consensus Standards," meaning it can be applied there.
Update on Cybersecurity: The inclusion of software as a medical device in the third edition of ISO 14971 (Annex F) introduces the topic of data security and cybersecurity for the first time. The Annex proposes standards for six important pillars that DTx must comply with: Security, Threat, Vulnerability, Confidentiality, Integrity, and Availability (of data).
A new regulatory benchmark for Digital Heath Applications: DiGA – Germany
The German Federal Government has recently injected some life into the rather sterile world of regulations and standards by introducing a new integrated set of laws in order to boost digitization in healthcare settings:
- Law for better coverage through digitization and innovation — Digital Supply Act (DGV) came into effect on 19 December 2019.
- Regulation on the procedure and requirements for testing the eligibility of digital health applications for reimbursement by the statutory health insurance scheme — Digital Health Applications Regulation (DiGAV).
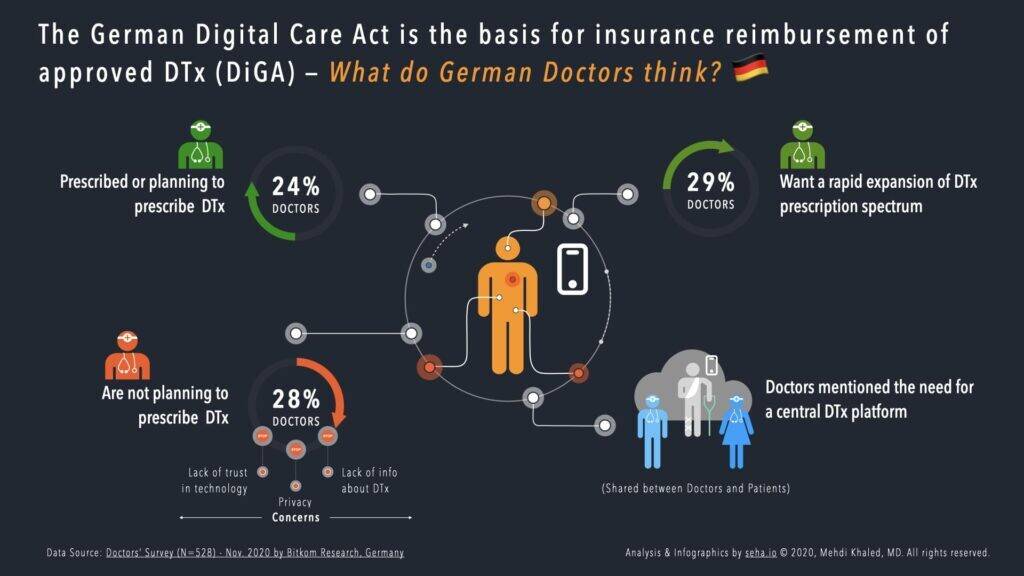
This uniquely innovative integration of laws and policies covers the full lifecycle of digital health applications (DTx) and creates a whole new market dynamic that's already making the German digital health ecosystem one of the fastest-growing markets today. These new dynamics have two majors impacts:
- Insured persons are entitled to a Digital Health Application (DiGA). A new procedure has been established to test the respective DiGA for safety, functional capability, quality, data security and data protection. After clearance, the DiGA is made available online in a corresponding directory for one year. It can be prescribed by doctors, downloaded by patients and reimbursed by the statutory health insurance. The DiGA providers have one year to prove that their products cost-effectively improve outcomes. This closed-loop process will help accelerate time-to-value and phase out less efficient solutions.
- Health insurance funds will be allowed to promote DTx innovations using up to two percent of their financial reserves for this purpose when acquiring investment assets by promoting DTx startups or participating in a health innovation fund (€200 million through 2024).
Finally, it is noteworthy that even though most DTx solutions on the market today do not require regulatory clearance, they all submit to the process of randomized clinical trials or observational studies. Notwithstanding, there's no linearity between the effectiveness of the DTx applications and its licensing status. In fact, being a non-licensed DTx doesn't reduce the value of the intervention as its efficacy is still backed by scientific evidence, albeit sometimes not independently validated. Many non-licensed DTx like Fastic, Sleepio and Happify have tens of thousands of downloads with equal numbers of documented, improved outcomes. Moreover, some non-licensed DTx like Big Health's Sleepio and Daylight have been successfully endorsed by the NHS based on their scores of sleep efficiency measures.
Critical Success Factors for DTx
Two facts fuel the current excitement about DTx in the digital health communities:
- The evidence DTx are truly transforming the way care is delivered, risks are managed and diseases are prevented.
- The potential lies ahead of this digital health segment, which is making its very first baby steps.
In order to deliver their full potential, it is therefore essential for the DTx community to stay focused on critical factors:
- Science-Guided Innovation: DTx application domains will have to take guidance from scientific evidence and be validated by independent, peer-reviewed processes. Initial validations followed by larger-scale cost-effectiveness studies will help identify superior DTx interventions, always and primarily for the patient's best interest.
- Data Privacy: Embedded privacy compliance in DTx solutions (GDPR, HIPAA and other regulations) ought to be a standard feature. DTx providers need to keep a lookout on legal changes and update their products accordingly.
- Information Security: As cyber criminals seek to take advantage of rapid digital health scale-up, it becomes imperative for DTx developers to adopt 'secure by design' principles. This also means thorough testing, documenting a security risk assessment, risk management, and imposing IT security requirements for the operating environment. Compliance never guarantees security, so if you're a DTx developer and want to tick the cybersecurity compliance box for your product to pass, you're probably not doing enough.
- Integrated Policies: Although still evolving, the new DTx market dynamics created by the German regulators represent a very encouraging new benchmark that levels the playing field.
- Clinical & Cost Effectiveness: With over 571 effectiveness studies published between 2007 and 2017 globally, the current trend is exponential. Moreover, the evidence standards framework for digital health technologies developed by UK-NICE represents a complete set of guidelines for digital developers, research funders and evaluators (insurances, commissioners, etc.). Evidence of DTx efficacy and effectiveness can take the form of a meta-analysis, a systematic review, a randomized control trial or an observational study. All are vital for the claims and cost-effectiveness validation which are some of the essential criteria for adopting DTx into the Standards of Care process.
- Adoption by clinicians: Initially integrated into the standard of care guidelines, scaling this adoption will require strong incentives and campaigns anchored in the evidence provided by the effectiveness studies and rewarded by a value-based care system. The fee per service care model will be counter-productive for DTx physician adoption in the long run.
- Adoption by patients: It is probably the most critical factor to consider, as it's where the rubber meets the road. Patient's acceptance and ultimately adoption will depend on many factors ranging from the user interface (minimal requirement to manually input data) to emotional activation and measurable outcomes. Obviously, smartphone and internet penetrations are expected to support further and accelerate this adoption.
DTx are transforming the way care is delivered, risks are managed and diseases are prevented
- Integration with value-based care (VBC) schemes: The success of digital therapies heavily hinges upon their data flows. For obvious continuity of care and data integrity reasons, DTx need to seek integration with care provider workflows before ultimately becoming part of a health system's VBC processes. This standard-based, secure step is made easy today, and products like One Drop and BlueStar are already heading in that direction.
- Reimbursement: A cornerstone for the scalability and sustainability of DTx interventions is their capacity to generate reimbursements through insurances. Although this is trending positively in some countries like Germany and the US, the pace of insurance adoption of DTx is inexistent to slow in many other countries. This adoption can be supported and accelerated by clinical effectiveness studies and a clear set of regulations.
- Industry Consolidation: The barriers to enter the DTx provider arena is markedly lower than those for drug research and development. This has democratized innovation and created a vibrant new ecosystem with a myriad of digital health startups and very keen investor communities. Although this is great for innovation quality and volumes, it is creating a fast-paced marketplace fragmentation. Moreover, the hefty new investment by big pharmaceutical and Med Tech companies in digital health capacity building in general, and DTx development in particular (so-called 'beyond the pill' solutions), will generate a mismatching set of market powers against the still fragile DTx startups. For fear of being driven into obsolescence, these startups need to regroup around each other and build new, strategic partnerships and alliances along two main business channels: B2B and B2C. This will fortify their position against the big players and accelerate their DTx adoption and a common quest for reimbursement.
The Future of DTx
Markets
As DTx markets mature, we should expect a consolidation through synergistic alliances (mainly between DTx companies) and mergers and acquisitions across the board. Moreover, the blurred line between wellness app and prevention DTx shall catalyze consolidations between players in both fields.
While North America emerged as a global leader for DTx in 2019 due to an exploding investment stream fuelled by DTx user adoption, Europe holds a stable second position as regulations and encourages investment incentives rollout. Notwithstanding, the Asia Pacific region is poised to witness the fastest growth through 2027 thanks to a vast consumer base along with a rising elderly population and high digital literacy levels.
Technology
There are two interesting technology-related areas to keep under the radar for DTx:
- DTx Development & Curation Platforms: These will be important for both speed to market and scale. Adopting these platforms will help DTx companies stay focused on their core business (science) and enable them to take advantage of a built-for-purpose infrastructure and security features. Accenture's Intient DTx Platform is certainly one of many to come.
- Emerging Technologies: The advances of DTx solutions will heavily depend on their capacity to harness the power of new technologies as these approach maturation. Smart Advisors, Affective Computing, Cognitive Computing, Biochips, Haptics, Volumetric and Holographic Displays, Natural Language Questions Answering are a few promising technologies that require a special place on the DTx roadmap.
Intervention Spectrum (Range of Field)
So far, limited to a few domains, as new technologies and new scientific evidence merge, the list of diseases and disorders tackled by DTx will exponentially expand. Mental Health is still a sweet spot for DTx and it will continue to breed more interventions in years to come. The next big impact domain for DTx will be chronic diseases and related risk factors such as obesity and smoking. For reference, the DTx Alliance keeps an updated overview of the therapeutic domains on its website. While conquering new intervention domains, digital therapies will reach a new level of maturity when they start adapting to various cultures and languages and developing personalized, accurate, predictive algorithm capabilities.
Multi-Condition Management (Depth of Field)
The reality in healthcare suggests that chronically ill patients very often have co-morbidities and other risk factors. In fact, the obese 62-year old smoker with essential hypertension, type II diabetes, bilateral glaucoma, and chronic lumbar pain is almost a stereotypical profile found in all GP waiting rooms today. Needless to say that however advanced some digital therapies may be today, they're only able to manage one condition at a time for any given patient. On top of his medication, it would therefore be counter-productive for our 62-year old patient to juggle between four different DTx Apps to manage each condition separately: SmokeFree to quit smoking, Fastic to manage his weight via intermittent fasting, MySugr to manage his diabetes, and Kaia Health's Motion Coach for his MSK condition. The real potential for next-generation DTx lies within their capacity to harness the power of emerging technologies and develop the ability to manage multiple conditions with high reliability of predictive value simultaneously.
Mehdi Khaled is the Managing Partner of Seha. He is a Medical Doctor specialized in Internal Medicine, a Software Engineer and a Digital Health strategy expert — driven by value-based technology. With over 25 years of hands-on experience in 40+ health systems worldwide and direct contributions in countless international digital health project implementations, he also advised top health executives over successful strategy practices and high-impact initiatives. The author declares no conflicting interests in the elaboration of this paper. More specifically, Mehdi declares not having any direct or indirect relationship of any kind with any of the companies or products mentioned in this article.






