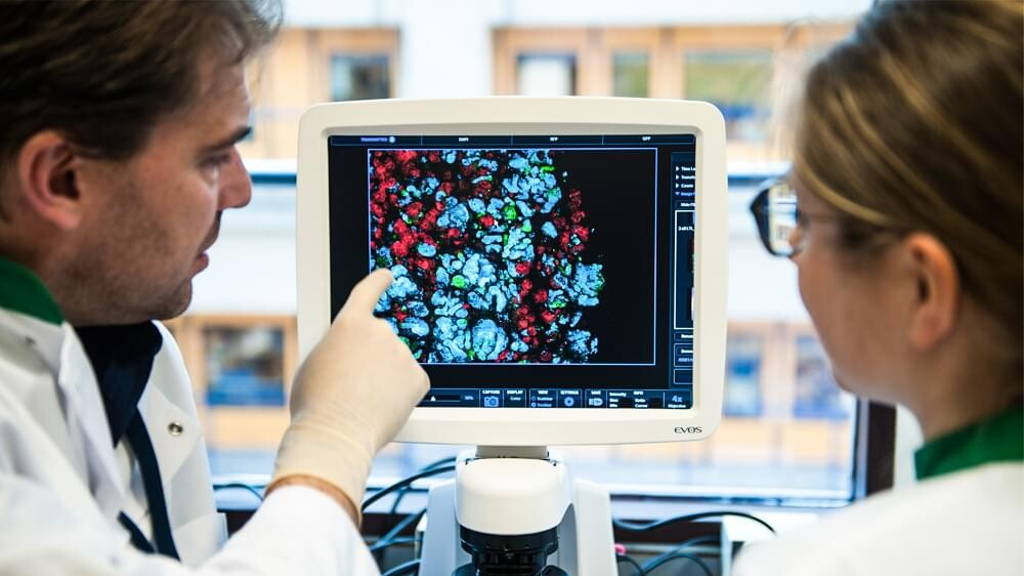
Regenerative medicine shows great promise. FDA is now catering to this promise, by making sure that the development and approval of regenerative medicine advanced therapies (RMAT) is measured by the right standards. It’s the Center for Biologics Evaluation and Research (CBER), the centre within FDA which regulates biological products for human use, which is especially gearing up to facilitate the development and approval of RMAT therapies, as defined and advanced by the 21st Century Cures Act.
Another part of the RMAT designation describes the fact that developers can get into early interactions with FDA staff.
The new policy gives FDA the right amount of flexibility to address these innovations, without compromising on standards. By doing this, the regulatory process for RMAT becomes more predictable. The agency might decide that small studies are enough for these therapies to receive the necessary approval. CBER is now working with organizations and partnerships to establish standards for defining and characterizing RMAT.
New legislation
The 21ste Century Cures Act launches the RMAT designation process, which is used to identify experimental RMAT products which require more attention from the FDA. According to the new legislation, FDA has to set standards on the development of these products and treatments and offer guidance to those developing them. RMAT includes cell therapies, therapeutic tissue engineering products, human cell and tissue products as well as genetically-modified cellular therapies and human tissues grown on scaffolds.Accelerating development
Developing regenerative medicines is hard enough as it is. Going through standard FDA reviews might slow the development of experimental products down significantly. The new legislation makes sure that this area of medicine gets the right treatment, by making priority review and accelerated approval possible for those therapies that to address unmet medical needs.Another part of the RMAT designation describes the fact that developers can get into early interactions with FDA staff.
The new policy gives FDA the right amount of flexibility to address these innovations, without compromising on standards. By doing this, the regulatory process for RMAT becomes more predictable. The agency might decide that small studies are enough for these therapies to receive the necessary approval. CBER is now working with organizations and partnerships to establish standards for defining and characterizing RMAT.