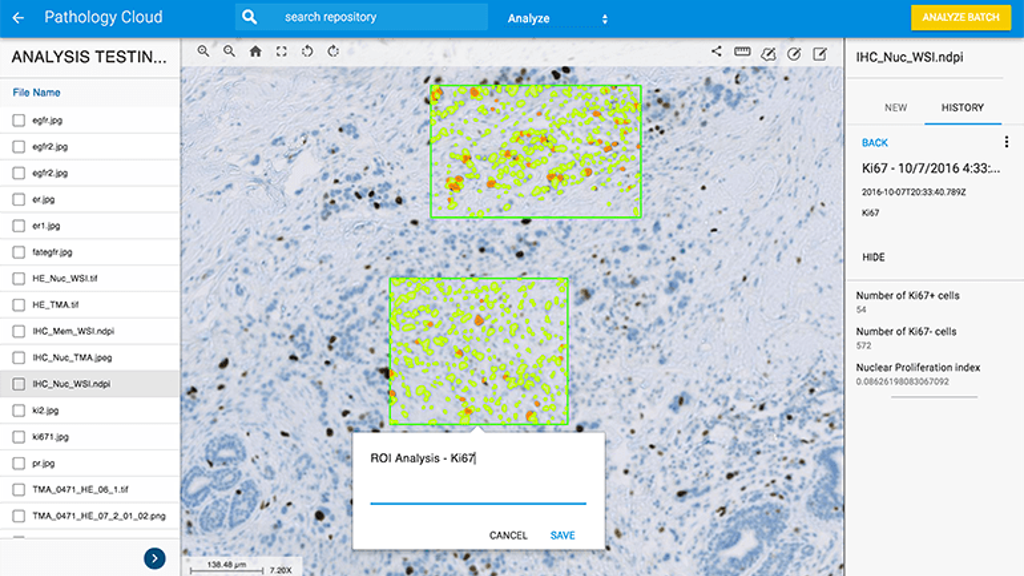
Since everything is done online and in the cloud allows researchers to analyze and share whole slide imagery and corresponding information, Medgadget writes. The new tools utilize deep learning neural network techniques that automatically analyze and quantify various tissue characteristics, cells, and biomarkers. Whole data sets can be processed without having to work through individual images, which should allow for more efficient research, according to Proscia in a blog concerning the new tools. The digital pathology startup says it is partnering with academic medical centers, contract research organizations, and biopharmaceutical companies to put pathology information to use in fighting cancer.
An overview of the new cloud based tools:
Stain Specific Quantification: Extraction of clinically significant features on whole slide images stained with the most common biomarkers, including Ki-67, HER2, and PD-L1, a key biomarker in immunotherapy research, discovery, and drug development. Auto-calibrates to adjust for inter-technician stain variability and sample quality, saving time and improving analysis accuracy.
Biomarker Agnostic Analysis: Biomarker-agnostic quantification of several dozen features of interest in the study of disease development, status, and progression. Useful for new biomarker development.
High-Throughput Dataset Manipulation: Manage and analyze datasets at scale, including applications for data integration, creation of control ground-truth to facilitate computed-based cancer region detection, large-scale data migration, and cross-platform integration.
Pathology is a subjective field that is dependent on how the eye of each pathologist views what appears in the microscope, says Dr. Clive Taylor, the principal investigator. “Defining how to measure and assess just how this process compares to a digital reading is complex. The design of this study holds the original diagnosis to be the truth and that provides us with a base to compare all readings by all pathologists in the study.”
Researcher Dr. Michael Feldman adds: “Proving that digital reads are not inferior to optical reads creates a foundation for a FDA submission that, if cleared, would allow for the shift to a digital operation that, in turn, may reap the additional benefits of collaboration, workflow efficiency and the use of precise tools for measurement and counting.”
An overview of the new cloud based tools:
Stain Specific Quantification: Extraction of clinically significant features on whole slide images stained with the most common biomarkers, including Ki-67, HER2, and PD-L1, a key biomarker in immunotherapy research, discovery, and drug development. Auto-calibrates to adjust for inter-technician stain variability and sample quality, saving time and improving analysis accuracy.
Biomarker Agnostic Analysis: Biomarker-agnostic quantification of several dozen features of interest in the study of disease development, status, and progression. Useful for new biomarker development.
High-Throughput Dataset Manipulation: Manage and analyze datasets at scale, including applications for data integration, creation of control ground-truth to facilitate computed-based cancer region detection, large-scale data migration, and cross-platform integration.
Digital versus traditional pathology diagnoses
Recently Royal Philips wrote in a press release that it successfully completed its well-controlled, multicenter pivotal validation study designed to compare diagnoses determined by pathologists from digital whole slide imaging to traditional optical diagnoses through a microscope. The study, which examined the viewing, reviewing and diagnosing of surgical pathology tissue slides from a range of tissues met its pre-specified endpoint.Pathology is a subjective field that is dependent on how the eye of each pathologist views what appears in the microscope, says Dr. Clive Taylor, the principal investigator. “Defining how to measure and assess just how this process compares to a digital reading is complex. The design of this study holds the original diagnosis to be the truth and that provides us with a base to compare all readings by all pathologists in the study.”
Researcher Dr. Michael Feldman adds: “Proving that digital reads are not inferior to optical reads creates a foundation for a FDA submission that, if cleared, would allow for the shift to a digital operation that, in turn, may reap the additional benefits of collaboration, workflow efficiency and the use of precise tools for measurement and counting.”